Introduction
The sickle cell solubility test, commonly known as “sickle prep” is a qualitative measure that relies on the property that deoxygenated hemoglobin S (HbS) is insoluble. Accordingly, blood samples drawn from individuals with circulating HbS appear cloudy when mixed with sodium hydrosulfite. 1 The sickle cell solubility test has been reported to be very sensitive (99%) and specific (99.9%) for detection of HbS. However, false negatives have been reported to occur in sickle cell disease (SCD) patients with severe anemia or high levels of hemoglobin F. 2,3 In those situations HbS levels may be very low and it remains unclear what level of HbS is required for the solubility test to be positive. To address this issue, we have examined the sickle cell solubility test in a cohort of SCD patients undergoing red cell exchange, acknowledging that in many of these patients, HbS levels fall below 15% when tested immediately after completing the exchange procedure.
Methods
118 patients with sickle cell anemia were screened based on outpatient visits to the Inova Adult Sickle Center from January 1, 2023 - March 31 st, 2023. Of these, 6 patients were excluded from analysis due to lack of recent sickle cell solubility test during this period. Of the total number of patients included in the analysis (n=112), 25 (22%) underwent red cell exchange during this period.
For each individual, the two most recent HbS percentages with the concurrently-determined sickle cell solubility result (positive or negative) were analyzed. We examined the data in the context of known genotype (HbSS, HbSC, HbSβ 0, HbSβ +positive). In patients undergoing red cell exchange, the most recent pre and post transfusion hemoglobin S percentage were collected.
Results:
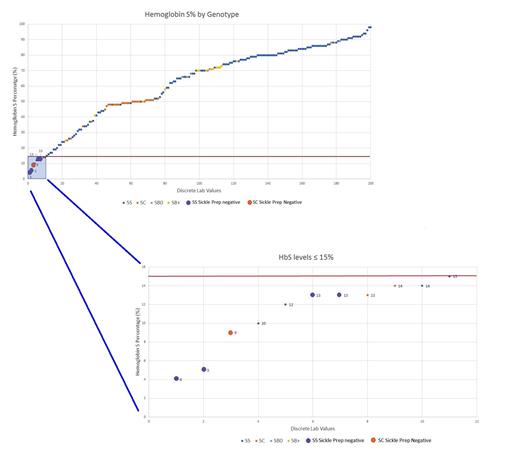
200 distinct HbS levels were collected and analyzed. Of those, 5/200 tested negative for the sickle cell solubility test, yielding an overall sensitivity rate of 98%. Of those 5 patients, 4/5 had received a red cell exchange during this period, and (1/5) had severe transfusion dependent anemia. Of those with a negative sickle cell solubility test, 4/5 patients had an SS genotype, and 1/5 had SC genotype. In patients who received red cell exchange, the sensitivity rate for the sickle cell solubility test is 84%.
For all patient samples with HbS >15%, the sickle solubility test was 100% sensitive. However, among the 11 samples with HbS levels below 15%, 5 were sickle prep negative thereby indicating a reduced sensitivity (55%) below that threshold. Nine (9) out of the 11 samples were of patients who had undergone red cell exchange.
Conclusion:
The sickle cell solubility is a very sensitive measure to establish the presence of HbS, but must be interpreted with caution in those with HbS concentrations of <15%. Because it is readily available (typically resulted within an hour) it may be of benefit in emergency departments for patients for whom there is question about the validity of the sickle cell diagnosis. At our institution, this has been a valuable addition to the initial management of patients for whom medical records are not available and who present with apparent acute vaso-occlusive crisis. We do not propose withholding analgesia for patients with a reported history of sickle cell disease pending this test, but rather recommend its use as a confirmatory test for patients presenting with a reported history of this disease.
References
1. Canning DM, Huntsman RG. An assessment of Sickledex as an alternative to the sickling test, J Clin Path, 1970, vol. 23 8(pg. 736-737)
2. Hicksg EJ, Griep JA, Nordschow CD. Comparison of results for three method of hemoglobin S identification. Clin Chem. 1973 May;19(5):533-5. PMID: 4703665.
3. Tubman VN, Field JJ. Sickle solubility test to screen for sickle cell trait: what's the harm? Hematology Am Soc Hematol Educ Program. 2015;2015:433-5. doi: 10.1182/asheducation-2015.1.433. PMID: 26637754.
Disclosures
Ershler:Global Blood Therapeutics: Other: Advisory board; Responder's office; Novartis: Other: Advisory board; Responder's office, Speakers Bureau; Pharmacosmos: Other: Advisory board, Speakers Bureau; Pfizer: Other: Advisory board, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal